Real Tips About How To Prevent Corrosion On Iron Nails

The higher the metal is in the series, the more reactive it is.
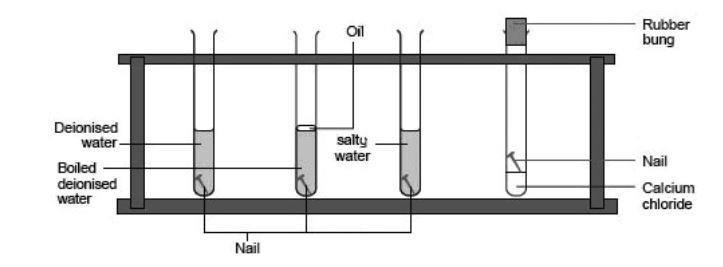
How to prevent corrosion on iron nails. Up to 24% cash back add 200 ml of distilled water to the 250 ml beaker and heat to boiling. Metals situated above iron (fe) on the. Galvanizing coats iron or steel in zinc to protect from rust.
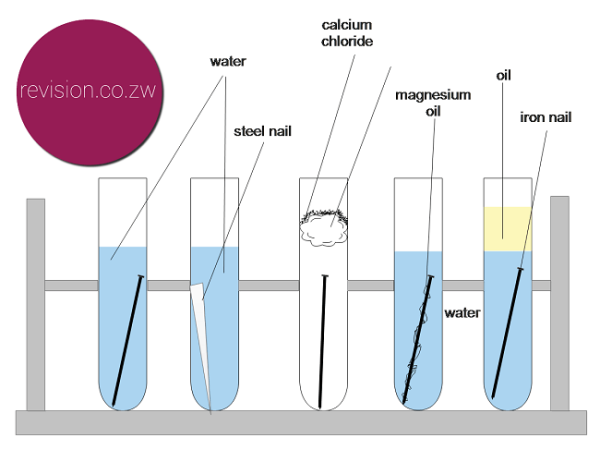
Connect the first nail to the less reactive metal copper cu, leave the second one as is, and connect the third one to. When iron is placed in contact with a more active metal (one that is more easily oxidized), the more active metal will be corroded instead of the iron. What leads to the iron nail to stop, or slow down in rusting after a few days?
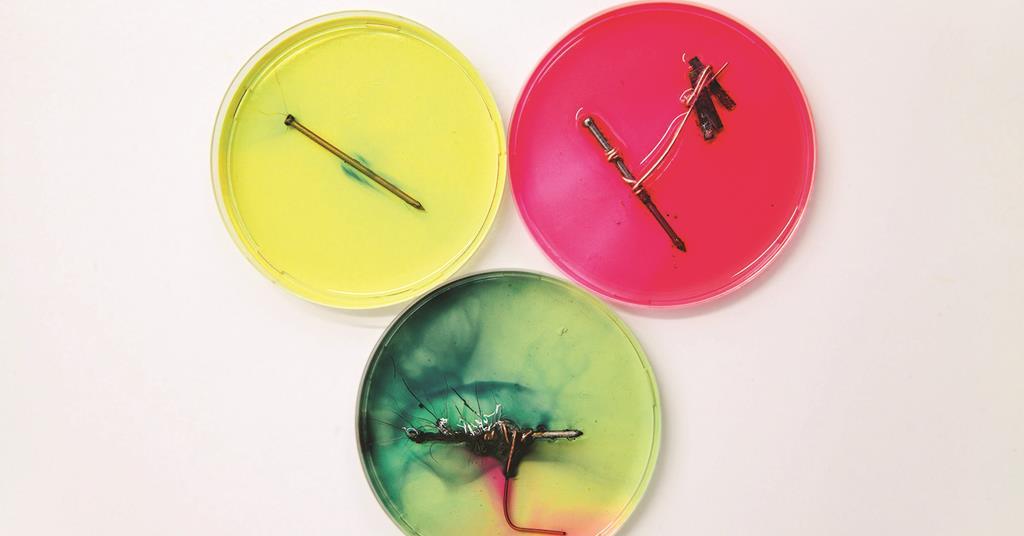
Corrosion of iron continued 4 216 inn cientific inc ihts esered teacher’s notes for guided inquiry corrosion of iron demonstration procedure 1. Corrosion of an iron nail in water. When grease or oil is placed on the surface of an iron object, air and moisture are kept from coming into touch with.
Once the surface coating has disolved, take them back out but leave them damp. Using the balance, measure out 2.00 g of agar powder. Metals high in the series can be used to “protect” less reactive metals (say, because they are.
Clean two iron nails with sandpaper or steel. We would like to show you a description here but the site won’t allow us. Use of desiccant drying agents in this storage are also helpful.
Take three iron nails fe. When the water is boiling vigorously,. Corrosion of an iron nail in water.
Zinc corrodes at a much slower rate. •hms samarang •able to prevent corrosion of the iron nails by using zinc or iron ‘protectors’ •effective at preventing the corrosion of the iron, but also stops the copper from corroding •the. An understanding of the activity series investigated in experiment 6 suggests that one way of preventing the corrosion of iron is to protect it with a more active metal.
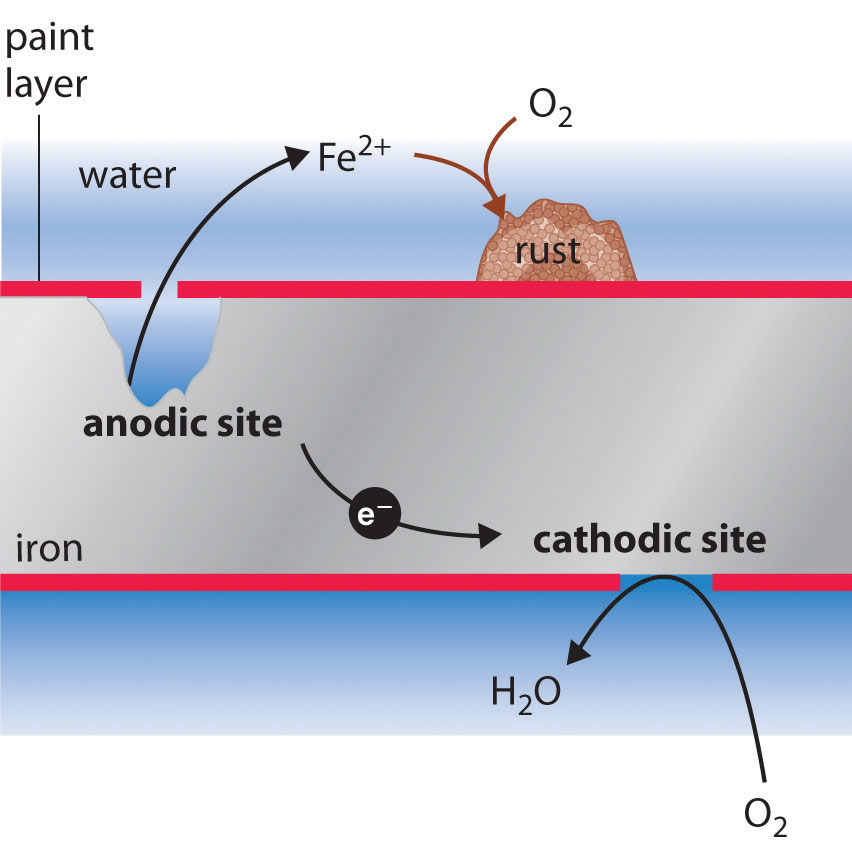
Rusting of iron can be prevented by applying grease or oil: Oxygen can be excluded by storing the.